上海金畔生物科技有限公司代理New England Biolabs(NEB)酶试剂全线产品,欢迎访问官网了解更多产品信息和订购。
产品信息
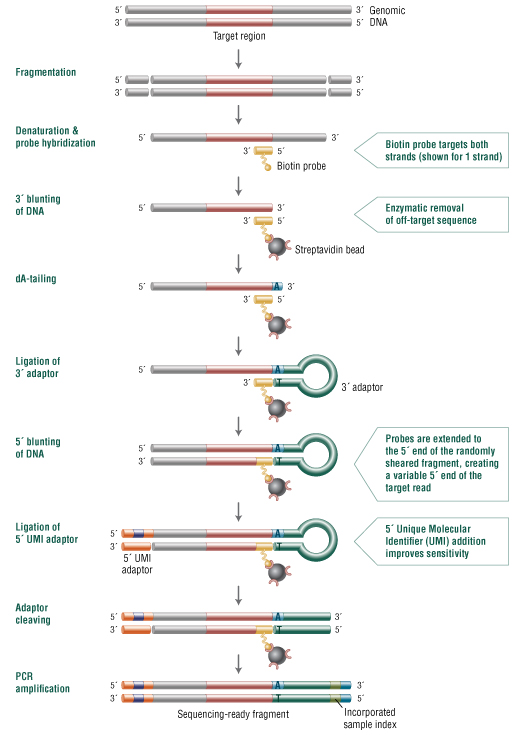
Target enrichment, coupled with next generation sequencing (NGS), enables high-throughput, deep sequencing of genomic regions of interest. NEBNext Direct is a novel, hybridization-based capture method offering significant advantages over traditional in-solution hybridization and multiplex PCR protocols.
In the NEBNext Direct target enrichment approach (Figure 1), enzymatically nicked or Covaris® sheared DNA is hybridized to biotinylated oligonucleotide baits that capture both strands of the target DNA and define the 3´ ends of the regions of interest. After hybridization, the bait-target hybrids are bound to streptavidin beads and any 3´ off-target sequence is removed enzymatically. This combination of hybridization with enzymatic removal of 3´ off-target sequence enables greater sequencing specificity relative to conventional hybridization-based enrichment methods. The trimmed targets are then converted into Illumina-compatible libraries that include a 12 bp unique molecular identifier (UMI) in the Illumina i5 index location and an 8 bp sample barcode in the Illumina i7 index location. The NEBNext Direct enrichment method can be performed within one to two days and is compatible with most automated liquid handling instruments.
The NEBNext Direct BRCA1/BRCA2 Panel is designed to enrich for the complete exonic content of the BRCA1 and BRCA2 genes. This kit contains the oligonucleotides, beads, enzymes and buffers required to convert the desired fragments into a sequence-ready library for next-generation sequencing on the Illumina platform and is designed for PE75 or PE150 sequencing.





Integrative Genome Viewer (IGV) plot showing the read coverage across exon 1 of the BRCA1 gene. 50 ng of DNA was enriched using the 17 kb NEBNext Direct BRCA1/BRCA2 Panel. Sequencing reads were generated on an Illumina MiSeq with 2×75 bp reads, an 8 bp sample ID, and 12 bp unique molecular identifier. Sequencing read alignments were performed with BWA-MEM, and PCR duplicates were filtered using the UMIs.
NEBNext Direct® BRCA1/BRCA2 Resources:
- GRCh37hg19 Bed Files for the NEBNext Direct® BRCA1/BRCA2 Panel
- GRCh38hg38 Bed Files for the NEBNext Direct® BRCA1/BRCA2 Panel
- Protocol for NEBNext Direct BRCA1/BRCA2 Panel (E6627)
- 产品类别:
- Discontinued (<3 years)
-
特性和用法
需要但不提供的材料
- Covaris® microTubes or plate
- 1X TE buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA)
- Molecular grade ethanol
- Molecular grade water
- 96-well PCR plates (or PCR strip tubes)
- Eppendorf® DNA LoBind® 2 ml tubes (VWR, cat#: 80077-234)
- Additional microcentrifuge or conical tubes to prepare master mixes
- 96-well plate magnet or PCR tube magnet
- Microcentrifuge tube magnet
- Agilent® High Sensitivity DNA Kit (Agilent, cat#: 5067-4626)
Required Equipment:- Covaris Focused-Ultrasonicator
- Thermocycler programmable to 100 μl
- Agilent Bioanalyzer® or similar instrument
-
优势和特性
应用特性
The NEBNext Direct BRCA1/BRCA2 Panel for Illumina® is designed to enrich for complete exonic content for BRCA1 and BRCA2 genes for next-generation sequencing on the Illumina platform. This kit contains the oligonucleotides, beads, enzymes and buffers required to convert the desired fragments into a sequence-ready library containing both sample indexes and unique molecular identifiers (UMI). The NEBNext Direct enrichment method can be performed within one day and is easily automated. Each kit component must pass rigorous quality control standards and is lot controlled both individually and as a set of reagents.
Lot Control: The lots provided in the NEBNext Direct BRCA1/BRCA2 Panel are managed separately and qualified by additional functional validation. Individual reagents undergo standard enzyme activity and quality control assays.
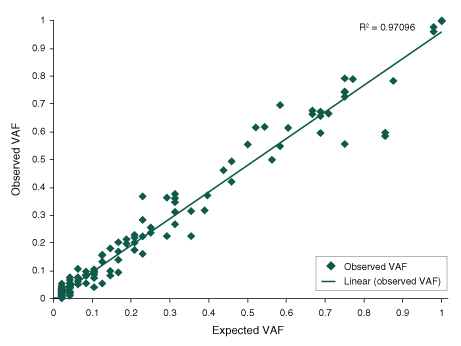
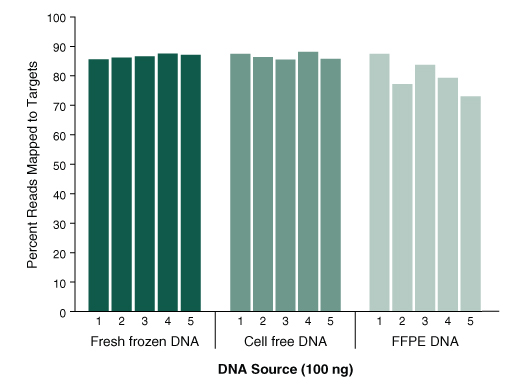
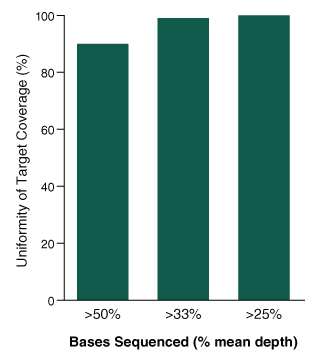
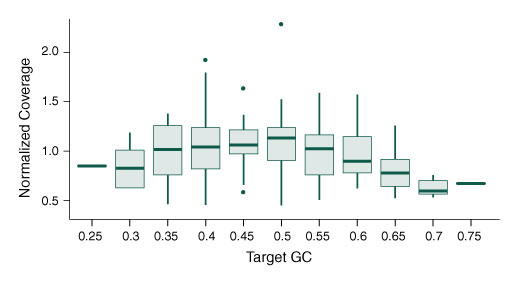
Functional Validation: Each set of reagents is functionally validated together through construction and sequencing of a target enriched DNA library on an Illumina sequencing platform. For 100 ng of NA19240 DNA input and an average coverage of 100X, 100% of the targets are covered.
Need a Bulk Order?
For larger volume requirements, customized and bulk packaging is available by purchasing through the Custom Solutions Team at NEB. Please contact custom@ neb.com for further information.
操作说明、说明书 & 用法
-
操作说明
- Protocol for NEBNext Direct BRCA1/BRCA2 Panel (E6627)
- Guidelines for Setting up PCR Reactions (E6627X only)
- Guidelines for Running Samples on the Illumina MiSeq (E6627)
-
说明书
产品说明书包含产品使用的详细信息、产品配方和质控分析。- manualE6627
-
使用指南
- GRCh37hg19 Bed Files for the NEBNext Direct® BRCA1/BRCA2 Panel
- GRCh38hg38 Bed Files for the NEBNext Direct® BRCA1/BRCA2 Panel
- Index Pooling Guideline for E6627
- Sample Sheet for E6627L with the index sequences that can be used in the Illumina® experiment Manager v4
- Sample Sheet for E6627L with the index sequences that can be used in the Illumina® experiment Manager v5
- Sample Sheet for E6627S with the index sequences that can be used in the Illumina® experiment Manager v4
- Sample Sheet for E6627S with the index sequences that can be used in the Illumina® experiment Manager v5
- Sample Sheet for E6627X with the index sequences that can be used in the Illumina® experiment Manager v4
- Sample Sheet for E6627X with the index sequences that can be used in the Illumina® experiment Manager v5
- Using Unique Molecular IDs with NEBNext Direct® – Data Usage Guideline Page
FAQs & 问题解决指南
-
FAQs
- Can NEBNext Direct be applied for genotyping applications?
- Are there sample sheets available to ensure sequencer runs are configured appropriately?
- What size should I fragment my DNA to?
- Can I repair my FFPE DNA using the NEBNext FFPE DNA Repair Mix (NEB #M6630)?
- How can I warm Bead Wash 1 (BW1) to dissolve any precipitate?
- If I have adaptor dimer in my finished library, can I perform another bead cleanup to remove it?
- How many times can the -20°C reagents be frozen and thawed?
- How many times can the 4°C reagents be brought to room temperature?
- My 4°C box accidentally froze, can I still use it?
- I accidentally stored the Sample Purification Beads and Bead Wash 1 (BW1) at 4°C. Can I still use them?
- What is the shelf life of this product?
- What method do you recommend for extracting genomic DNA and FFPE DNA before use with the NEBNext Direct Panel?
- Can shearing be done using Covaris®?
- What is the recommended method for quantitating my input DNA?
- If my DNA input material amount is high, should I reduce the number of PCR cycles?
- If my input amount is low, should I dilute the adaptors?
- What type and how much starting material do I need to use when preparing libraries using the NEBNext Direct Panel?
- Which magnets (2 ml and 200 µl sizes) do you recommend?
- Is it ok to leave bead separations for longer than 15 seconds?
- I used Bead Wash 1 (BW1) when I should have used Bead Wash 2 (BW2) during the Post-reaction Wash. Can I do another wash with Bead Wash 2 (BW2) to save my sample?
- I used Bead Wash 2 (BW2) when I should have used Bead Wash 1 (BW1) during the Post-reaction Wash. What should I do?
- Does incomplete removal of Bead Wash 1 prior to adding Bead Wash 2 inhibit the next step?
- Can I use any Q5® formulation with this kit?
- How many sequencing reads per sample do you recommend using with the NEBNext Direct BRCA1/BRCA2 Panel?
-
How many/ few samples can I pool together for my sequencing run?
- Are the indexes in E7000S/ E6627S/E6631S (D01-D08) the same sequences as the first 8 indexes (D01-D08) in NEB #E7000L/ E6627L/E6631L?
- If my FFPE DNA is fragmented, should I do a shearing step?