- 产品特性
- 相关资料
- Q&A
- 参考文献
NASH细胞核受体
非酒精性脂肪肝(NASH/NAFLD)研究用核受体
了解核受体如何参与非酒精性脂肪性肝病(NAFLD)或非酒精性脂肪性肝炎(NASH)的发病机制,可能为肝病新疗法的研发提供新的方向。在药物和治疗发现中,最常见的核受体是由配体激活的核受体,包括过氧化物酶体增殖物激活受体α(PPARα)、孕烷X受体(PXR)和组成型雄甾烷受体(CAR),它们是最早被确定的化学毒物反应的关键调节受体。使用小鼠疾病模型和人体样本的大量研究表明,这些受体和PPARβ/δ、PPARγ、法尼醇X受体(FXR)和肝X受体(LXR)等其他受体,通过调节肠-肝-脂肪轴在维持营养/能量稳态方面发挥着关键作用。
NAFLD与核受体功能的改变和肠-肝轴的紊乱有着密切联系。这些紊乱包括肥胖、肝脏脂质代谢异常、炎症增加和胰岛素抵抗。核受体与新陈代谢、炎症和再生紧密联系,对于研究NAFLD/NASH等肝病的生理学和病理学有着重要意义。经证实,核受体的调节可以减少肝脂肪变性、炎症、胰岛素抵抗、纤维化和肥胖,有望成为强力且有效的治疗靶点。
◆NASH 细胞核受体
目前研究表明,NASH药物、治疗的研发与核受体激活有关,具体包含以下核受体:
● PPARα (NR1C1)
● PPARγ (NR1C3)
● PPARβ/δ (NR1C2)
● FXR (NR1H4)
● LXRα (NR1H3)
● LXRβ (NR1H2)
● VDR (NR1I1)
● PXR (NR1I2)
● CAR (NR1I3)
● TRα (NR1A1)
● RARα (NR1B1)
● RARβ (NR1B2)
● RORγ (NR1F3)
INDIGO Biosciences的NASH细胞核受体分析产品是基于细胞的报告分析系统。其特点是使用了CryoMite™ 工艺进行制备的工程核受体特异性报告细胞。解冻后,报告细胞可立即使用。报告细胞包含健康、分裂的哺乳动物细胞的细胞质和核环境中表达的人核受体,可筛选待测化合物的激动剂或拮抗剂活性。
INDIGO Biosciences致力于为非酒精性脂肪肝研究提供合适的核受体。INDIGO可提供清晰明确的单受体或全面板筛选结果, 让客户在整个研发过程中作出正确的决策。通过采用发光法和INDIGO的 CryoMite™ 保存工艺,INDIGO的核受体产品在化合物功效、效能和选择性的研究上可为您提供批间可重复的实验结果。
◆非酒精性脂肪肝
非酒精性脂肪性肝病(NAFLD)的特点为肝脏炎症的发生以及肝细胞中由非过度饮酒引起的多余脂肪堆积。肝脏中含有一些天然脂肪,但若肝脏脂肪的重量含量超过5-10%,则被称为脂肪肝(脂肪变性)。NAFLD是发达国家最常见的肝脏疾病,仅在美国就有超过1亿成人和儿童受到影响。研究表明,NAFLD在拉丁美洲血统的人群中最为常见,但也发生在所有种族和民族的人群中。
非酒精性脂肪性肝炎(NASH)是NAFLD的严重表现形式,是肝硬化和肝癌的主要原因。
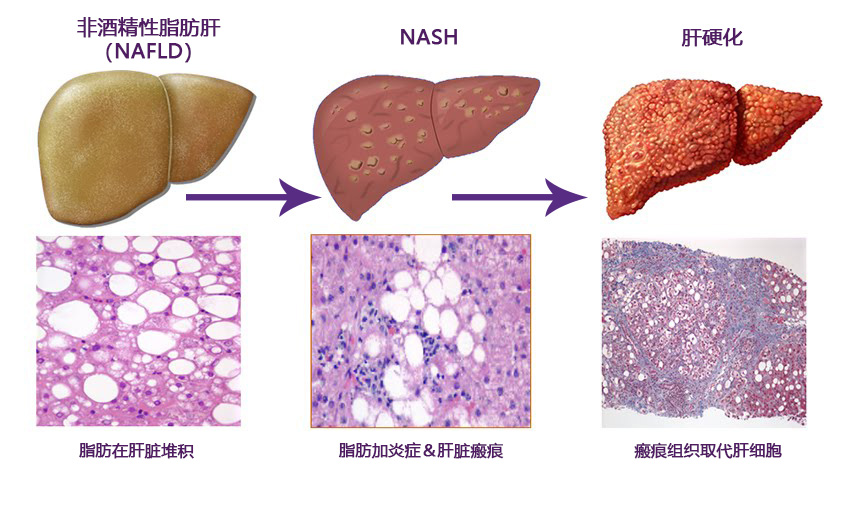
大多数NAFLD患者没有出现任何症状,部分人可能会出现疲劳、右上腹不适或轻度黄疸。目前,估计约20%的NAFLD患者同时患有NASH。肝活检是被广泛接受并明确用于区分NASH与其他形式肝病的唯一诊断手段。通常在常规血液检查中发现肝功能异常后再进行肝活检诊断。常见的异常指标有肝酶升高和肝脏超声显示脂肪变性。
据P&S Market Research的研究,截至2017年3月,超50种潜在的肝病治疗候选药物正在研发中。因肝病存在病因学未知、其病理生理学复杂和治疗成本高昂等因素,了解肝病细胞核受体如何反应有助进一步研究肝病。
相关文献
|
1. |
Liver Foundation 全文 |
|
2. |
DDNews Special Focus on Nonalcoholic Steatohepatitis; Date of publication: March 2017; DDNews 全文 |
|
3. |
Nonalcoholic Steathohepatits Therapeutics Market; Date of publication: 2017; P&S Market Research 全文 |
|
4. |
Treatment of non-alcoholic fatty liver disease; Date of publication: May 2006; BMJournal 全文 |
|
5. |
The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease; Date of publication: March 2005; Clinics in Liver Disease; vol 8, issue 3 全文 |
| 产品编号 | 产品名称 | 产品规格 | 产品等级 |
