上海金畔生物科技有限公司代理New England Biolabs(NEB)酶试剂全线产品,欢迎访问官网了解更多产品信息和订购。
产品信息
Luna 探针一步法 RT-qPCR 4X 预混液,含 UDG(无 ROX)用于探针法实时荧光定量检测靶标 RNA。首先反转录酶将 RNA 转化为 cDNA,然后 DNA 聚合酶对 cDNA 进行扩增,完成靶标的实时荧光定量 PCR(qPCR),整个过程在单管内一次性完成。基于探针法的 qPCR / RT-qPCR 原理是利用聚合酶的 5´ → 3´ 外切酶活性,将淬灭的目标特异性探针切断发出荧光,并实时监测荧光值的增加,以测量每个循环中的 DNA 扩增。在荧光信号显著超过背景荧光的位置,可以确定 Cq 值。Cq 值可用于评估两个或多个样品的靶基因相对丰度。
Luna 探针一步法 RT-qPCR 预混液,含 UDG(无 ROX)为 4X 浓度,因此在靶标 RNA 量极低的应用中(例如:病原体检测),可提高样品加入量。通过对 5 个靶标进行不同浓度的线性、灵敏度检测,显示该产品在多重检测中性能更优。该预混液将一步法 RT-qPCR 所需组分整合到单个试管中,包括:Luna 温启动反转录酶、Taq 热启动 DNA 聚合酶、dNTP 和小鼠 RNase 抑制剂。Taq 热启动 DNA 聚合酶与新型温启动反转录酶联合使用,可通过基于适配体的可逆抑制来双重控制酶的活性。这种温控活化机制可避免热循环前的非特异扩增,从而让室温建立体系更安全。经改造的 Luna 温启动反转录酶具有更高的热稳定性,最佳反应温度为 55°C。该剂型不含参比染料,适用于不需要 ROX 参比染料的仪器(如需 ROX 矫正,可添加 ROX)。
RT-qPCR 的高灵敏度使得避免 DNA 污染尤为重要。由于先前扩增产物可作为后续反应的底物,因此反应体系加入 dUTP 和热敏 UDG 可防止模板残留污染。热敏 UDG 超过 50°C时会完全失活,因此对 qPCR 性能没有影响。
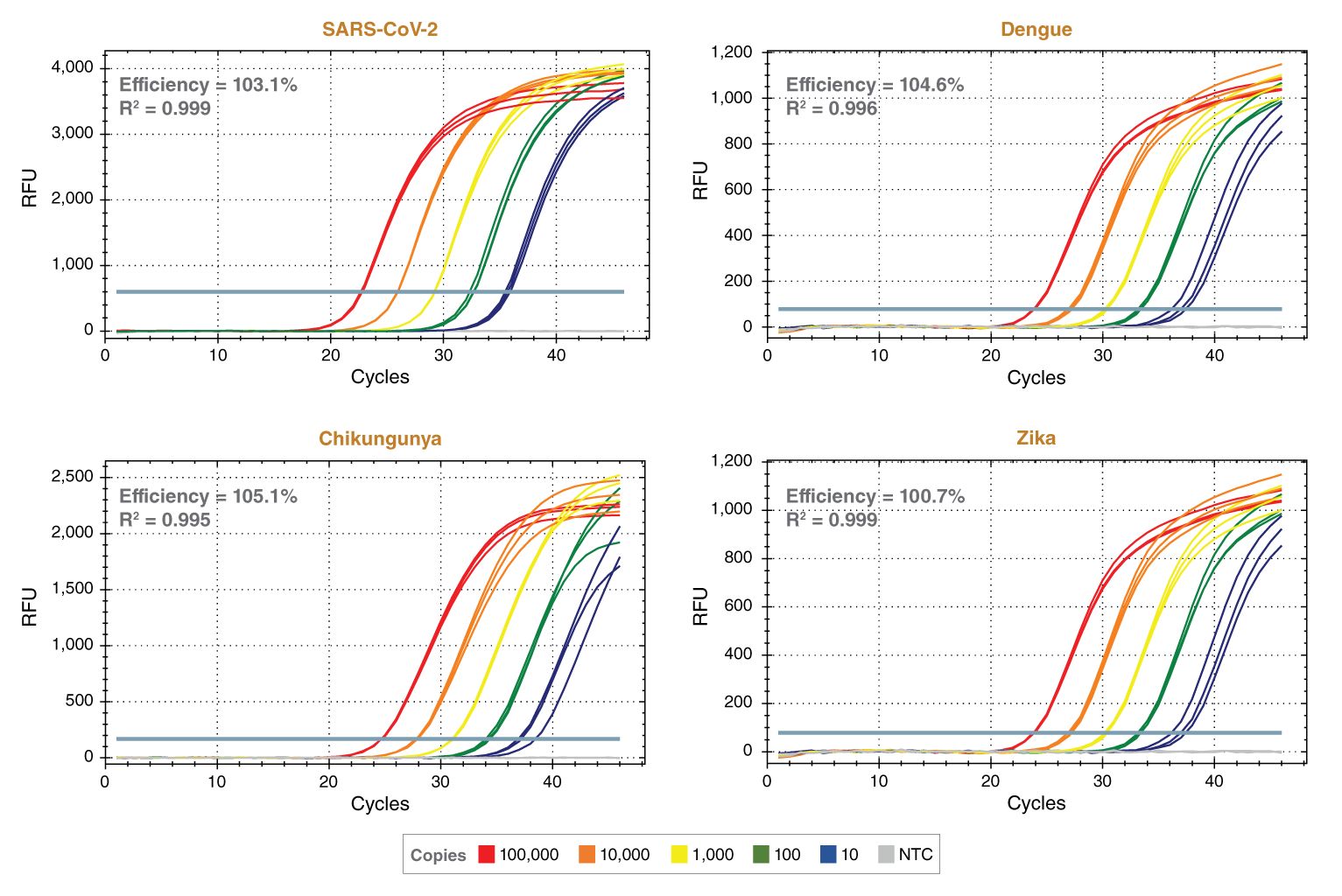
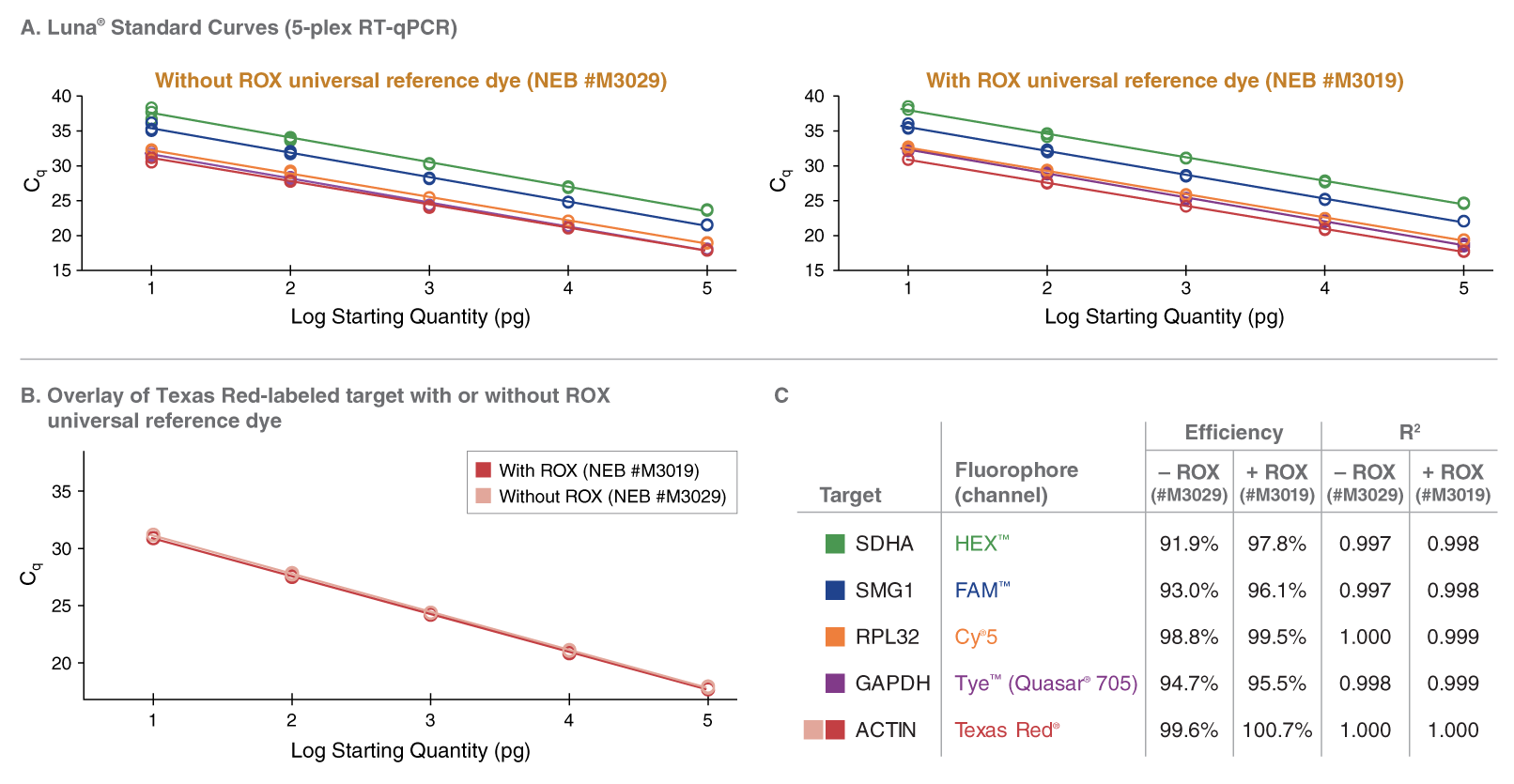
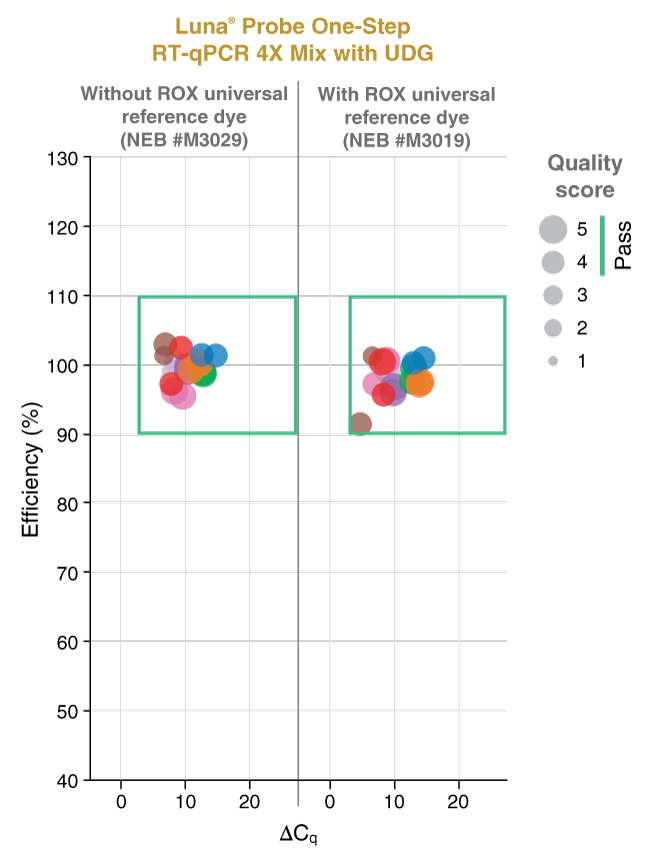
该预混液包含无荧光的可见蓝色可见示踪染料,有助于透明管加样。该示踪染料与 qPCR 常用荧光基团的光谱没有重叠,也不会干扰实时检测。

- 产品类别:
- Luna® qPCR & RT-qPCR Products,
- PCR, qPCR & Amplification Technologies Products
- 应用:
- cDNA Synthesis
-
产品组分信息
本产品提供以下试剂或组分:
NEB # 名称 组分货号 储存温度 数量 浓度 -
M3029S -20 Luna® Probe One-Step RT-qPCR 4X Mix with UDG (No ROX) M3029SVIAL -20 2 x 0.5 ml 4 X Nuclease-free Water B1502AVIAL -20 2 x 1.5 ml Not Applicable
-
M3029L -20 Luna® Probe One-Step RT-qPCR 4X Mix with UDG (No ROX) M3029LVIAL -20 2 x 1.25 ml 4 X Nuclease-free Water B1502AVIAL -20 4 x 1.5 ml Not Applicable
-
M3029E -20 Luna® Probe One-Step RT-qPCR 4X Mix with UDG (No ROX) M3029EVIAL -20 1 x 10 ml 4 X Nuclease-free Water B1502EVIAL -20 1 x 25 ml Not Applicable
-
-
相关产品
相关产品
- Monarch® 总 RNA 小量提取试剂盒
- M3019 Luna Probe One-Step RT-qPCR 4X Mix with UDG
- Luna® SARS-CoV-2 多重 RT-qPCR 检测试剂盒
- Luna® 通用探针一步法 RT-qPCR 试剂盒
- Luna® 探针一步法 RT-qPCR 试剂盒(无 ROX)
操作说明、说明书 & 用法
-
操作说明
- Protocol for Luna® Probe One-Step RT-qPCR 4X Mix with UDG (No ROX) (NEB #M3029)
-
应用实例
- Optimized conditions for the CDC Influenza SARS-CoV-2 (Flu SC2) Multiplex Assay using Luna One-Step RT-qPCR Reagents
FAQs & 问题解决指南
-
FAQs
- How do I use qPCR to determine the concentration of my material?
- Can I set up my Luna® RT-qPCR at room temperature?
- Should I use probe- or dye-based detection for my qPCR assays?
- How should I design primers for Luna® qPCR?
- How long should my amplicon be for qPCR?
- Why is the Luna® qPCR Mix blue? Will this dye interfere with detection?
- Can I run the Luna® qPCR Mix on my qPCR instrument?
- Can I use fast instrument settings with the Luna® qPCR Mix?
- Do I need to add ROX?
- What RNA samples can be used in RT-qPCR with the Luna® Mix?
- How much primer and probe should I use with the Luna® Universal Probe RT-qPCR Kit?
- Can I use shorter cycling times?
- How much RNA template should I use in my RT-qPCR reaction?
- Can I run multiplex reactions with the Luna® Probe One-Step RT-qPCR Kits? Do I need to change my reaction conditions?
- Can I use a ROX-labeled probe with the Luna® Probe Mixes that contain a universal ROX reference dye?
- Can alternative probe based detection strategies be used with the Luna® Probe Mix?
- What temperature should I use for cDNA synthesis with Luna® RT-qPCR kits?
- Can I use longer targets in one-step RT-qPCR?
- Is the Monarch Total RNA Miniprep Kit compatible with Luna RT-qPCR Reagents?
- Are the Monarch RNA Cleanup Kits (NEB # T2030, #T2040, #T2050) compatible with Luna RT-qPCR reagents?
- What are the differences between the Luna One-Step RT-qPCR products (NEB #E3006, #E3007, #M3019, #M3029 and #L4001)?
- Other companies recommend a 2-min incubation at 25°C for carryover prevention, is a 30-sec incubation at 25°C sufficient?
- How can I perform a no-RT Control?
- Can I use the Luna Probe RT-qPCR mixes for viral detection?
质控、安全 & 法规
-
质控分析
每一新批次的 NEB 产品都要经过质控,以满足指定的质量标准,对特定产品的质量标准和每一批次的检测数据可以通过产品说明表格、COA、产品信息卡或者产品手册进行查阅或下载。关于 NEB 产品质控的详细信息,可从此处查阅。 -
产品说明与变更通知
产品说明表包含产品的储存温度、有效期和规格,这些文件的命名规则如下:[货号]_[规格]_[版本]- M3029S_L_E_v2
-
CoA 文件包含单个批次产品的储存温度、有效期和质控,这些文件的命名规则如下: [货号]_[规格]_[版本]_[Lot#]
- M3029E_v1_10122696
- M3029L_v1_10120297
- M3029S_v1_10120296
- M3029L_v1_10137516
- M3029L_v1_10141863
- M3029E_v1_10143761
- M3029L_v1_10145088
- M3029L_v1_10146243
- M3029L_v1_10146533
- M3029S_v1_10147178
- M3029E_v1_10148078
- M3029S_v1_10148704
- M3029L_v1_10150265
- M3029E_v1_10158367
- M3029S_v1_10159691
- M3029L_v1_10159786
- M3029E_v1_10167650
- M3029S_v1_10166370
- M3029E_v1_10171897
- M3029L_v2_10176923
- M3029S_v2_10178550
- M3029E_v2_10178547
- M3029S_v2_10208044
- M3029E_v2_10219884
- M3029L_v2_10215744
- M3029E_v2_10233815
- M3029L_v2_10233298
-
SDS
以下 SDS 文件可以帮助您安全地使用该产品-
Luna® 探针一步法 RT-qPCR 4X 预混液,含 UDG(无 ROX)
-
Nuclease-free Water
-
Luna® 探针一步法 RT-qPCR 试剂盒(无 ROX) |NEB酶试剂 New England Biolabs
上海金畔生物科技有限公司代理New England Biolabs(NEB)酶试剂全线产品,欢迎访问官网了解更多产品信息和订购。
产品信息
Luna 探针一步法 RT-qPCR 试剂盒(无 ROX)经过优化,适用于采用水解探针法的靶标 RNA 序列实时定量。一步法 RT-qPCR 为 RNA 检测和定量提供了一种便捷、强大的方法。在同一管中,RNA 首先由反转录酶转化为 cDNA,然后使用 DNA 依赖型 DNA 聚合酶扩增 cDNA,从而可以进行 qPCR 定量。探针法 qPCR/RT-qPCR 原理是利用聚合酶的 5´→3´ 核酸外切酶活性,将淬灭的目标特异性探针切断发出荧光,并实时监测荧光值的增加,以测量 PCR 每个循环中的 DNA 扩增。通过荧光信号显著超过背景荧光所经历的循环数,可以确定 Cq 值。Cq 值可用于评估两个或多个样品之间的靶基因相对丰度,或者参考通过已知浓度样品绘制的标准曲线,对未知样品绝对定量。
在 Luna 探针一步法 RT-qPCR 试剂盒(无 ROX)中,联合使用 Taq 热启动 DNA 聚合酶与新型 WarmStart 反转录酶,通过适配体可逆抑制来双重控制酶活性。这种温控活化机制可避免热循环前的非特异扩增,从而让在室温下建立体系更安全。经改造的 Luna WarmStart 反转录酶的热稳定性比许多反转录酶更高,其最佳反应温度为 55℃。对于困难靶标/模板,最多可将反转录步骤的温度升至 60℃,且不会影响 Luna 性能。
注意:为确保 Luna WarmStart 反转录酶完全激活,建议温育温度不低于 50℃。
Luna 探针一步法 RT-qPCR 试剂盒(无 ROX)浓度为 2X,包含 Taq 热启动 DNA 聚合酶、dNTP 和所有必需的缓冲液成分。配方中不含参比染料,与不需要 ROX 的仪器兼容(如果需要 ROX 校正,可自行添加 ROX 染料)。反应预混液包含可防止交叉污染的 dUTP,以及有助于观察反应体系建立的非荧光可见示踪染料。该示踪染料与 qPCR 常用荧光团的光谱没有重叠,因此不会干扰实时检测。
Luna WarmStart 反转录酶预混液的浓度为 20X,其中包含 Luna WarmStart 反转录酶以及用于防止 RNA 降解的小鼠 RNase 抑制剂(详情请见产品手册中的模板制备)。适用于各种 RNA 样品类型(总 RNA、poly(A)-RNA 等)和来源。
.
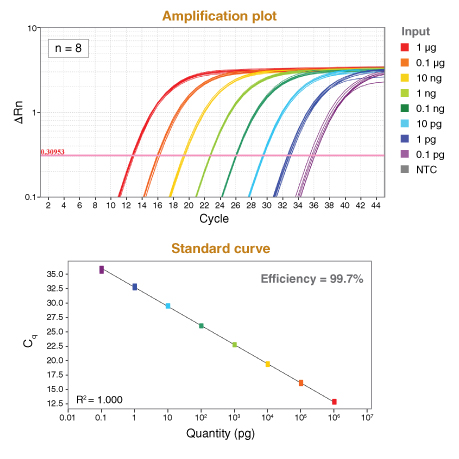
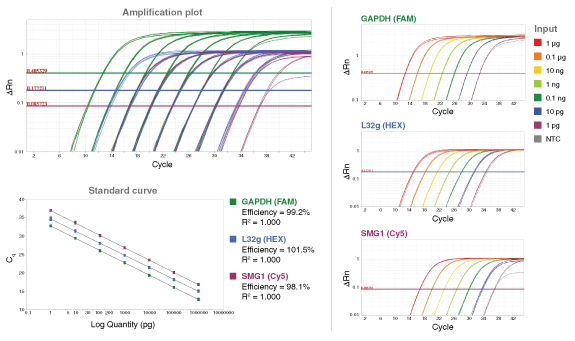
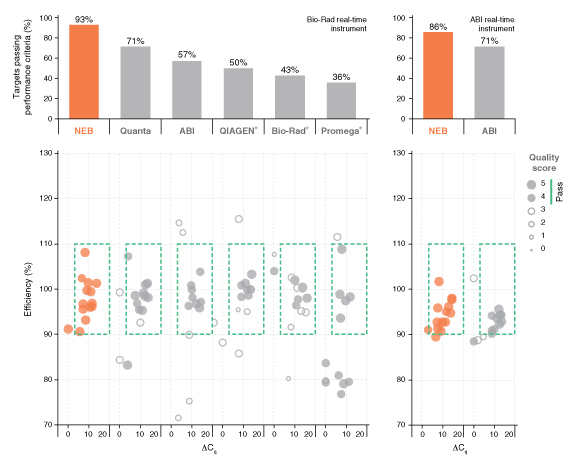
详细了解 qPCR/RT-qPCR 综合测试和“dots in boxes”数据可视化分析方法。
*数据来源于 Luna 通用探针一步法 RT-qPCR 试剂盒(NEB #E3006)。Luna 探针一步法 RT-qPCR 试剂盒(无 ROX)(NEB #E3007)的性能与之相同。
- 产品类别:
- Luna® qPCR & RT-qPCR Products,
- PCR, qPCR & Amplification Technologies Products
- 应用:
- qPCR & RT-qPCR,
- PCR,
- DNA Amplification, PCR & qPCR
-
试剂盒组成
本产品提供以下试剂或组分:
NEB # 名称 组分货号 储存温度 数量 浓度 -
E3007E -20 Luna® WarmStart® RT Enzyme Mix M3002EVIAL -20 2 x 1.25 ml 20 X Luna® Probe One-Step Reaction Mix (No ROX) M3007EVIAL -20 1 x 25 ml 2 X Nuclease-free Water B1502EVIAL -20 1 x 25 ml Not Applicable
-
-
相关产品
相关产品
- 南极热敏 UDG
- Luna® 通用一步法 RT-qPCR 试剂盒
- Luna® 通用 qPCR 预混液
- Luna® 通用探针法 qPCR 预混液
-
注意事项
- Primer Design
The use of qPCR primer design software (e.g., Primer3) maximizes the likelihood of amplification success while minimizing nonspecific amplification and primer dimers. Targets with balanced GC/AT content (40–60%) tend to amplify most efficiently. Where possible, enter sufficient sequence around the area of interest to permit robust primer design and use search criteria that permit cross-reference against relevant sequence databases (to avoid potential off-target amplification). It is advisable to design primers across known RNA splicing sites in order to prevent amplification from genomic DNA. - Primer and Probe Concentrations
For most targets, a final concentration of 400 nM (each primer) will provide optimum performance. If needed, primer concentrations can be optimized between 100–900 nM. Probe should be included at 200 nM for best results. Probe concentration can be optimized in the range of 100–500 nM. - Multiplexing
When determining which fluorophores to include in a multiplex reaction, be sure to choose compatible reporter dyes and quenchers (e.g., those that can be accommodated by the chosen real-time instrument with minimal overlap in fluorescence spectra). For ROX-dependent instruments, avoid ROX-labeled probes. Include 400 nM of forward and reverse primers and 200 nM probe for each target to be detected in the reaction. For targets that differ significantly in abundance, use of a lower primer concentration (e.g. 200 nM) for the more abundant target(s) is recommended. Adjust concentrations if necessary based on performance (primer 100–900 nM, probe 100–500 nM). When loading a qPCR protocol onto the real-time instrument, be sure to select the appropriate optical channels, as some instruments have a single channel recording mode that would prevent multiplex data collection and analysis. The functionality of the primer and probe sets should be tested individually before attempting a multiplex reaction. - Amplicon Length
To ensure successful and consistent qPCR results, it is important to maximize PCR efficiency. An important aspect of this is the design of short PCR amplicons (typically 70–200 bp). Some optimization may be required for targets that exceed that range. - Template Preparation and Concentration
Luna RT-qPCR is compatible with RNA samples prepared through typical nucleic acid purification methods. Prepared RNA should be stored in an EDTA-containing buffer (e.g., 1X TE) for long-term stability, and dilutions should be freshly prepared for a qPCR experiment in either TE or water. Note that the quality of RNA templates can greatly affect RT-qPCR efficiency. RNA should be handled with appropriate precautions to prevent RNase or DNase contamination. Use of nuclease-free water (provided) is strongly recommended. Where useful, RNA may be treated with DNase I to remove contaminating genomic DNA.
Generally, a useful concentration of standard and unknown material will be in the range of 108 copies to 10 copies. Note that for dilutions in the single-copy range, some samples will contain multiple copies and some will have none, as defined by the Poisson distribution. For total RNA, Luna One-Step Kits can provide linear quantitation over an 8-order input range of 1 μg – 0.1 pg. For most targets, a standard input range of 100 ng – 10 pg total RNA is recommended. For purified mRNA, input of ≤ 100 ng is recommended. For in vitro-transcribed RNA, input of ≤ 109 copies is recommended. - ROX Reference Dye
Some real-time instruments recommend the use of a passive reference dye (typically ROX) to overcome well-to-well variations that could be caused by machine limitations such as “edge effect”, bubbles, small differences in volume, and autofluorescence from dust or particulates in the reaction. However, ROX normalization does little to the variations caused by pipetting errors of templates/primers, heterogeneous mixing, and evaporation/condensation issues.
A universal passive reference dye is included in the following Luna® qPCR products: Luna Universal qPCR Master Mix (NEB #M3003), Luna Universal Probe qPCR Master Mix (NEB #M3004), Luna Universal One-Step RT-qPCR Kit (NEB #E3005), and Luna Universal Probe One-Step RT-qPCR Kit (NEB #E3006). These products support broad instrument compatibility (High-ROX, Low-ROX, ROX-independent) so no additional ROX is required for normalization.
The Luna Probe One-Step RT-qPCR Kit (No ROX) (E3007) contains no reference dye and is compatible with any instrument that does not require ROX. If ROX normalization is needed, ROX can be added. Please refer to instrument manufacturer’s instructions for greater details.
- Carryover Contamination Prevention
RT-qPCR is an extremely sensitive method, and contamination in new RT-qPCR assays with products from previous amplification reactions can cause a variety of issues, such as false positive results and a decrease in sensitivity. The best way to prevent this “carryover” contamination is to practice good laboratory procedures and avoid opening the reaction vessel post amplification. However, to accommodate situations where additional anti-contamination measures are desired, Luna qPCR mixes contains a mixture of dUTP/dTTP that results in the incorporation of dU into the DNA product during amplification. Pretreatment of qPCR/RT-qPCR experiments with uracil DNA glycosylase (UDG) will eliminate previously-amplified uracil-containing products by excising the uracil base to produce a non-amplifiable DNA product. The use of a thermolabile UDG is important, as complete inactivation of the UDG is required to prevent destruction of newly synthesized qPCR products.To enable carryover prevention, 0.025 units/μl Antarctic Thermolabile UDG (NEB #M0372) should be added to the reaction mix. To maximize elimination of contaminating products, set up the qPCR experiments at room temperature or include a 10 minute incubation step at 25°C before the initial denaturation step.
- Reaction Setup and Cycling Conditions
Due to dual hot-start feature of Luna One-Step Kits, it is not necessary to set up reactions on ice or preheat the thermocycler prior to use.
For 96-well plates, a final reaction volume of 20 μl is recommended.
For 384-well plates, a final reaction volume of 10 μl is recommended.
When programming instrument cycling conditions, ensure a plate read is included at the end of the extension step, and a denaturation (melt) curve after cycling is complete to analyze product specificity.
Amplification for 40 cycles is sufficient for most applications, but for very low input samples 45 cycles may be used.
- Primer Design
操作说明、说明书 & 用法
-
操作说明
- Luna® Probe One-Step RT-qPCR Kit (No ROX) Protocol (NEB# E3007)
-
说明书
产品说明书包含产品使用的详细信息、产品配方和质控分析。- manualE3007
-
应用实例
- High-throughput qPCR and RT-qPCR Workflows Enabled by Beckman Coulter Echo Acoustic Liquid Handling and NEB Luna Reagents
- Facilitating Detection of SARS-CoV-2 Directly from Patient Samples: Precursor Studies with RT-qPCR and Colorimetric RT-LAMP Reagents
FAQs & 问题解决指南
-
FAQs
- How do I use qPCR to determine the concentration of my material?
- Can I set up my Luna® RT-qPCR at room temperature?
- What is the difference between probe- and dye-based versions of the Luna® qPCR Mixes?
- Should I use probe- or dye-based detection for my qPCR assays?
- How should I design primers for Luna® qPCR?
- How long should my amplicon be for qPCR?
- Why is the Luna® qPCR Mix blue? Will this dye interfere with detection?
- Can I use fast instrument settings with the Luna® qPCR Mix?
- Do I need to add ROX?
- How many dilutions should I use to make a standard curve?
- Why does NEB recommend 40-45 cycles?
- Does the Luna® qPCR Mix contain dUTP? Can I use carryover contamination prevention methods?
- Can I run multiplex reactions with the Luna® Probe One-Step RT-qPCR Kits? Do I need to change my reaction conditions?
- Can I use a ROX-labeled probe with the Luna® Probe Mixes that contain a universal ROX reference dye?
- Can alternative probe based detection strategies be used with the Luna® Probe Mix?
- How much primer and probe should I use with the Luna® Universal Probe RT-qPCR Kit?
- How do I choose between one-step RT-qPCR and two-step RT-qPCR?
- What RNA samples can be used in RT-qPCR with the Luna® Mix?
- How much RNA template should I use in my RT-qPCR reaction?
- Can I use longer targets in one-step RT-qPCR?
- What temperature should I use for cDNA synthesis with Luna® RT-qPCR kits?
- Should I include a no Luna® WarmStart® Enyzme Mix control (-RT Control)?
- Is the Monarch Total RNA Miniprep Kit compatible with Luna RT-qPCR Reagents?
- Are the Monarch RNA Cleanup Kits (NEB # T2030, #T2040, #T2050) compatible with Luna RT-qPCR reagents?
- Can I use shorter cycling times?
- What are the differences between the Luna One-Step RT-qPCR products (NEB #E3006, #E3007, #M3019, #M3029 and #L4001)?
- Can I run the Luna® qPCR Mix on my qPCR instrument?
- Can I use the Luna Probe RT-qPCR mixes for viral detection?
Luna® 通用 qPCR 预混液 |NEB酶试剂 New England Biolabs
上海金畔生物科技有限公司代理New England Biolabs(NEB)酶试剂全线产品,欢迎访问官网了解更多产品信息和订购。
产品信息
Luna 通用 qPCR 预混液是经优化的 2X 反应预混液,可以用于对靶标 DNA 序列进行实时 qPCR 检测和定量,适用于使用大多数实时荧光定量 qPCR 仪器的 SYBR®/FAM 通道。该预混液包含热启动 Taq DNA 聚合酶,还添加了独特的惰性参比染料,该染料可与多种 qPCR 仪器兼容(包括需要高 ROX 或低 ROX 参比信号的仪器)。独具特色的是:预混液还包含可防止交叉污染的 dUTP,以及有助于观察反应体系建立的非荧光可见示踪染料。该染料的光谱与 qPCR 荧光染料不重叠,因此不会影响到实时检测数值。
该预混液浓度为 2X,包含 DNA 扩增和定量所需的所有 PCR 组分(引物和 DNA 模板除外)。使用 Luna qPCR 配合已有的商品化的 qPCR 测定引物,可对基因组 DNA 或感兴趣的 cDNA 进行定量检测。
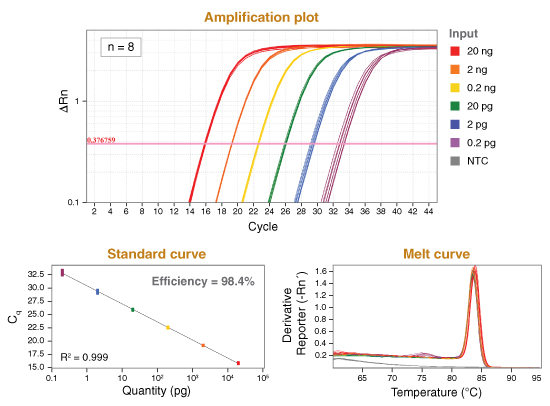
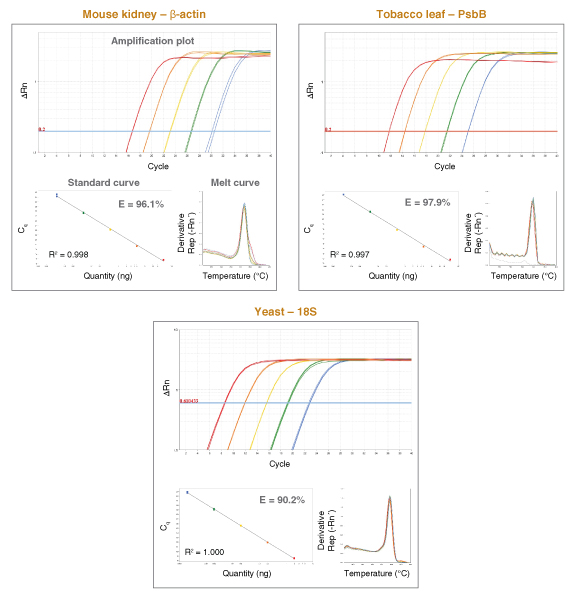
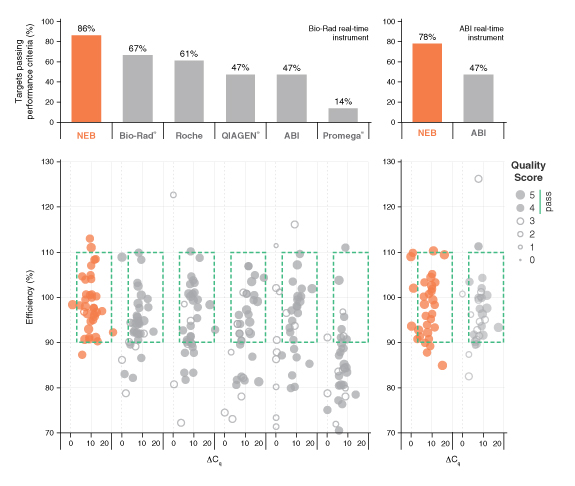
详细了解 qPCR/RT-qPCR 综合测试和“dots in boxes”数据可视化分析方法

- 产品类别:
- Luna® qPCR & RT-qPCR Products,
- PCR, qPCR & Amplification Technologies Products
- 应用:
- qPCR & RT-qPCR,
- DNA Amplification, PCR & qPCR
-
产品组分信息
本产品提供以下试剂或组分:
NEB # 名称 组分货号 储存温度 数量 浓度 -
M3003V -20 Luna® Universal qPCR Master Mix M3003VVIAL -20 1 x 1 ml 2 X
-
M3003S -20 Luna® Universal qPCR Master Mix M3003SVIAL -20 2 x 1 ml 2 X
-
M3003L -20 Luna® Universal qPCR Master Mix M3003SVIAL -20 5 x 1 ml 2 X
-
M3003X -20 Luna® Universal qPCR Master Mix M3003SVIAL -20 10 x 1 ml 2 X
-
M3003E -20 Luna® Universal qPCR Master Mix M3003EVIAL -20 1 x 25 ml 2 X
-
-
相关产品
相关产品
- 南极热敏 UDG
- Luna® 通用探针法 qPCR 预混液
- Luna® 探针一步法 RT-qPCR 试剂盒(无 ROX)
- Luna® 通用一步法 RT-qPCR 试剂盒
- Luna® 通用探针一步法 RT-qPCR 试剂盒
- LunaScript™ 反转录 SuperMix 试剂盒
操作说明、说明书 & 用法
-
操作说明
- Luna® Universal qPCR Master Mix Protocol (M3003)
- Protocol for Two-step RT-qPCR using the LunaScript® RT SuperMix Kit (NEB #E3010) and the Luna® Universal qPCR Master Mix (NEB #M3003) or Luna Universal Probe qPCR Master Mix (NEB #M3004)
-
说明书
产品说明书包含产品使用的详细信息、产品配方和质控分析。- manualM3003
-
使用指南
- Optimization Tips for Luna® qPCR
-
应用实例
- High-throughput qPCR and RT-qPCR Workflows Enabled by Beckman Coulter Echo Acoustic Liquid Handling and NEB Luna Reagents
FAQs & 问题解决指南
-
FAQs
- How do I use qPCR to determine the concentration of my material?
- Can I set up my Luna® qPCR at room temperature?
- What is the difference between probe- and dye-based versions of the Luna® qPCR Mixes?
- Should I use probe- or dye-based detection for my qPCR assays?
- How should I design primers for Luna® qPCR?
- How long should my amplicon be for qPCR?
- Why is the Luna® qPCR Mix blue? Will this dye interfere with detection?
- Can I run the Luna® qPCR Mix on my qPCR instrument?
- Can I use fast instrument settings with the Luna® qPCR Mix?
- Do I need to add ROX?
- How many dilutions should I use to make a standard curve?
- Why does NEB recommend 40-45 cycles?
- Does the Luna® qPCR Mix contain dUTP? Can I use carryover contamination prevention methods?
- Why do I have multiple peaks in my melt curve?
- How can I distinguish non-template amplification (NTC) from real products?
- Why do I see amplification curves in my NTC samples?
- What samples can be used in qPCR with the Luna® Mix?
- Can I use cDNA? Does it matter how I make it?
- How much template material can I use in Luna® qPCR?
- How much primer should I use for the Luna® Universal qPCR Master Mix?
- Can I use shorter cycling times?
- What is the fluorescent, double-stranded DNA binding dye in the Luna® qPCR master mix?
-
问题解决指南
- Luna® qPCR Troubleshooting Guide
Luna® 通用探针法 qPCR 预混液 |NEB酶试剂 New England Biolabs
上海金畔生物科技有限公司代理New England Biolabs(NEB)酶试剂全线产品,欢迎访问官网了解更多产品信息和订购。
产品信息
探针法定量 PCR(qPCR)原理是利用聚合酶的 5´→3´ 核酸外切酶活性,将淬灭的靶基因特异性探针切断,使其发出荧光,并实时监测释放出的荧光,以测量每个 PCR 循环中的 DNA 扩增。通过检测荧光信号显著超过本底信号时的循环数,可确定 Cq 值。Cq 值可用于评估评估两个或两个以上的样本之间的相对丰度,也通过梯度稀释已知浓度样品所绘制的标准曲线,对样本绝对定量。
Luna 通用探针法 qPCR 预混液是经优化的 2X 反应预混液,适用于水解探针法对靶标 DNA 序列进行实时 qPCR 检测和定量分析。该预混液包含热启动 Taq DNA 聚合酶,还添加了独特的惰性参比染料,该染料可与多种 qPCR 仪器兼容(包括需要高 ROX 或低 ROX 参比信号的仪器)。独具特色的是:预混液还包含可防止交叉污染的 dUTP,以及有助于观察反应体系建立的非荧光可见示踪染料。该染料的光谱与 qPCR 荧光染料不重叠,因此不会影响到实时检测数值。
该预混液浓度为 2X,包含 DNA 扩增和定量所需的所有 PCR 组分(引物/探针和 DNA 模板除外)。使用 Luna qPCR 试剂配合商品化的 qPCR 检测引物/探针,可以对基因组 DNA 或感兴趣的 cDNA 进行定量。
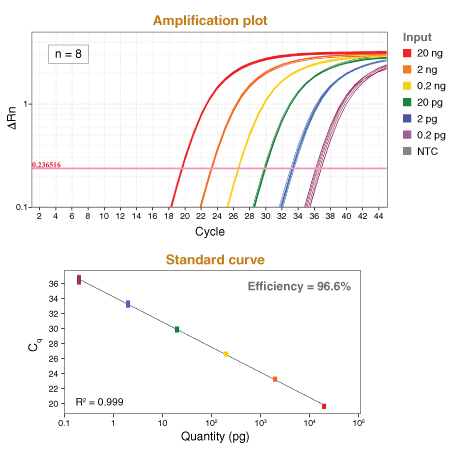
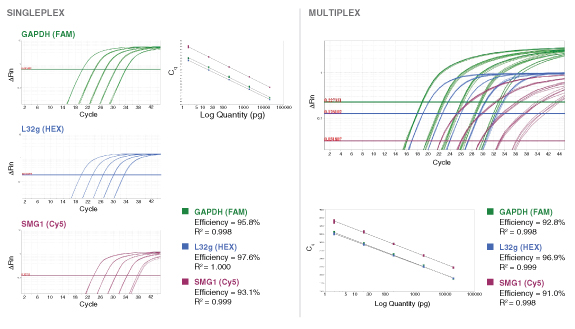
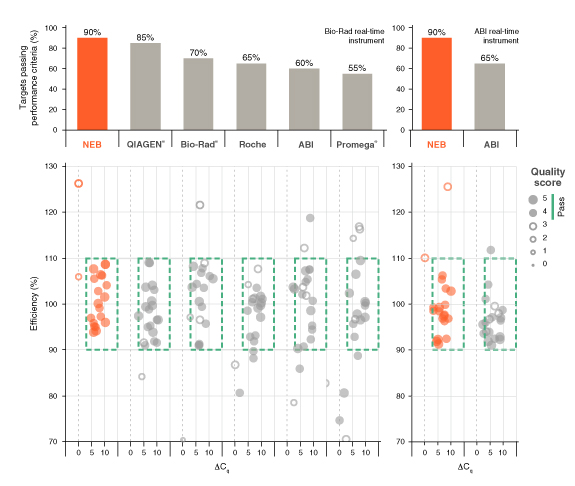
详细了解 qPCR/RT-qPCR 综合测试和“dots in boxes”数据可视化分析方法

- 产品类别:
- Luna® qPCR & RT-qPCR Products,
- PCR, qPCR & Amplification Technologies Products
- 应用:
- qPCR & RT-qPCR,
- DNA Amplification, PCR & qPCR
-
产品组分信息
本产品提供以下试剂或组分:
NEB # 名称 组分货号 储存温度 数量 浓度 -
M3004V -20 Luna® Universal Probe qPCR Master Mix M3004VVIAL -20 1 x 1 ml 2 X
-
M3004S -20 Luna® Universal Probe qPCR Master Mix M3004SVIAL -20 2 x 1 ml 2 X
-
M3004L -20 Luna® Universal Probe qPCR Master Mix M3004SVIAL -20 5 x 1 ml 2 X
-
M3004X -20 Luna® Universal Probe qPCR Master Mix M3004SVIAL -20 10 x 1 ml 2 X
-
M3004E -20 Luna® Universal Probe qPCR Master Mix M3004EVIAL -20 1 x 25 ml 2 X
-
-
相关产品
相关产品
- 南极热敏 UDG
- Luna® 通用 qPCR 预混液
- Luna® 探针一步法 RT-qPCR 试剂盒(无 ROX)
- Luna® 通用一步法 RT-qPCR 试剂盒
- Luna® 通用探针一步法 RT-qPCR 试剂盒
- LunaScript™ 反转录 SuperMix 试剂盒
-
注意事项
- Assay Design
The use of qPCR primer design software (e.g., Primer3) maximizes the likelihood of amplification success while minimizing nonspecific amplification and primer dimers. Targets with balanced GC/AT content (40–60%) tend to amplify efficiently. Where possible, enter sufficient sequence around the area of interest to permit robust primer design and use search criteria that permit cross-reference against relevant sequence databases (to avoid potential off-target amplification). For cDNA targets, it is advisable to design primers across known splicing sites in order to prevent amplification from genomic DNA. Conversely, primers designed to target intronic regions can ensure amplification exclusively from genomic DNA. - Primer and Probe Concentration
For most targets, a final concentration of 400 nM for each primer will provide optimum performance. If needed, primer concentrations can be optimized between 200–900 nM. Probe should be included at 200 nM for best results. Probe concentration can be optimized in the range of 100–500 nM if optimization of performance or target fluorescence level is desired. - Multiplexing
To detect or quantitate multiple targets in the same Luna reaction, select different fluorophores corresponding to separate detection channels of the real-time instrument. Include 400 nM of forward and reverse primer and 200 nM probe for each target to be detected in the reaction, and adjust concentrations if necessary based on performance (primer 200–900 nM, probe 100–500 nM). When loading qPCR protocol onto the real-time instrument, be sure to select the appropriate optical channels, as some instruments have a single channel recording mode that would prevent multiplex data collection and analysis. For ROX-dependent instruments, avoid ROX-labeled probes. The functionality of the primer and probe sets should be tested individually before attempting a multiplex reaction. When determining which fluorophores to include in a multiplex reaction, be sure to choose compatible reporter dyes and quenchers that have well separated fluorescence spectra or exhibit minimal overlap. - Amplicon Length
To ensure successful and consistent qPCR results, it is important to maximize PCR efficiency. An important aspect of this is the design of short PCR amplicons (typically 70–200 bp). Some optimization may be required (including the use of longer extension times), for targets that exceed that range. - Template Preparation and Concentration
Luna qPCR is compatible with DNA samples prepared through typical nucleic acid purification methods. Prepared DNA should be stored in an EDTA-containing buffer (e.g., 1X TE) for long-term stability, and dilutions should be freshly prepared for a qPCR experiment by dilution into either TE or water.
Generally, a useful concentration of standard and unknown material will be in the range of 106 copies to 1 copy. For gDNA samples from large genomes (e.g., human, mouse) a range of 50 ng–1 pg of gDNA is typical. For small genomes, adjust as necessary using 106 –1 copy input as an approximate range. Note that for single copy dilutions, some samples will contain multiple copies and some will have none, as defined by the Poisson distribution.
For cDNA, use the product of a reaction containing 1 μg–0.1 pg starting RNA. cDNA does not need to be purified before addition to the Luna reaction but should be diluted at least 1:10 into the qPCR. - ROX Reference Dye
Some real-time instruments recommend the use of a passive reference dye (typically ROX) to overcome well-to-well variations that could be caused by machine limitations such as “edge effect”, bubbles, small differences in volume, and autofluorescence from dust or particulates in the reaction. However, ROX normalization does little to the variations caused by pipetting errors of templates/primers, heterogeneous mixing, and evaporation/condensation issues.
A universal passive reference dye is included in the following Luna® qPCR products: Luna Universal qPCR Master Mix (NEB #M3003), Luna Universal Probe qPCR Master Mix (NEB #M3004), Luna Universal One-Step RT-qPCR Kit (NEB #E3005), and Luna Universal Probe One-Step RT-qPCR Kit (NEB #E3006). These products support broad instrument compatibility (High-ROX, Low-ROX, ROX-independent) so no additional ROX is required for normalization.
The Luna Probe One-Step RT-qPCR Kit (No ROX) (E3007) contains no reference dye and is compatible with any instrument that does not require ROX. If ROX normalization is needed, ROX can be added. Please refer to instrument manufacturer’s instructions for greater details.
- Carryover Contamination Prevention
qPCR is an extremely sensitive method, and contamination in new qPCR assays with products from previous amplification reactions can cause a variety of issues such as false positive results and a decrease in sensitivity. The best way to prevent this “carryover” contamination is to practice good laboratory procedures and avoid opening the reaction vessel post amplification. However, to accommodate situations where additional anti-contamination measures are desired, the Luna Universal Probe qPCR Master Mix contains a mixture of dUTP/dTTP that results in the incorporation of dU into the DNA product during amplification. Pretreatment of qPCR experiments with uracil DNA glycosylase (UDG) will eliminate previously-amplified uracil-containing products by excising the uracil base to produce a non-amplifiable DNA product. The use of a thermolabile UDG is important, as complete inactivation of the UDG is required to prevent destruction of newly synthesized qPCR products.To enable carryover prevention, 0.025 units/μl Antarctic Thermolabile UDG (NEB #M0372) should be added to the reaction mix. To maximize elimination of contaminating products, set up the qPCR experiments at room temperature or include a 10 minute incubation step at 25°C before the initial denaturation step.
- Reaction Setup and Cycling Conditions
Due to the hot start nature of the polymerase, it is not necessary to preheat the thermocycler prior to use or set up reactions on ice.
For 96-well plates, we recommend a final reaction volume of 20 μl.
For 384-well plates, a final reaction volume of 10 μl is recommended.
When programming instrument cycling conditions, ensure a plate read is included at the end of the extension step, and a denaturation (melt) curve after cycling is complete to analyze product specificity.
Amplification for 40 cycles is sufficient for most applications, but for very low input samples 45 cycles may be used.
- Assay Design
操作说明、说明书 & 用法
-
操作说明
- Luna® Universal Probe qPCR Master Mix Protocol (M3004)
- Protocol for Two-step RT-qPCR using the LunaScript® RT SuperMix Kit (NEB #E3010) and the Luna® Universal qPCR Master Mix (NEB #M3003) or Luna Universal Probe qPCR Master Mix (NEB #M3004)
-
说明书
产品说明书包含产品使用的详细信息、产品配方和质控分析。- manualM3004
-
使用指南
- Optimization Tips for Luna® qPCR
-
应用实例
- Probe-based qPCR Probe Compatibility and Multiplexing with Luna Universal Probe qPCR Master Mix
- High-throughput qPCR and RT-qPCR Workflows Enabled by Beckman Coulter Echo Acoustic Liquid Handling and NEB Luna Reagents
- Multiplex real-time PCR detection of monkeypox virus using Luna® qPCR Reagents
FAQs & 问题解决指南
-
FAQs
- How do I use qPCR to determine the concentration of my material?
- Can I set up my Luna® qPCR at room temperature?
- What is the difference between probe- and dye-based versions of the Luna® qPCR Mixes?
- Should I use probe- or dye-based detection for my qPCR assays?
- How should I design primers for Luna® qPCR?
- How long should my amplicon be for qPCR?
- Why is the Luna® qPCR Mix blue? Will this dye interfere with detection?
- Can I run the Luna® qPCR Mix on my qPCR instrument?
- Can I use fast instrument settings with the Luna® qPCR Mix?
- Do I need to add ROX?
- How many dilutions should I use to make a standard curve?
- Why does NEB recommend 40-45 cycles?
- Does the Luna® qPCR Mix contain dUTP? Can I use carryover contamination prevention methods?
- Can I run multiplex reactions with the Luna® Universal Probe Master Mix? Do I need to change my reaction conditions?
- Can I use a ROX-labeled probe with the Luna® Probe Mixes that contain a universal ROX reference dye?
- Can alternative probe based detection strategies be used with the Luna® Probe Mix?
- What samples can be used in qPCR with the Luna® Mix?
- Can I use cDNA? Does it matter how I make it?
- How much template material can I use in Luna® qPCR?
- How much primer and probe should I use with the Luna® Universal Probe qPCR Master Mix?
- Can I use shorter cycling times?
-
问题解决指南
- Luna® qPCR Troubleshooting Guide
