上海金畔生物科技有限公司代理New England Biolabs(NEB)酶试剂全线产品,欢迎访问官网了解更多产品信息和订购。
产品信息
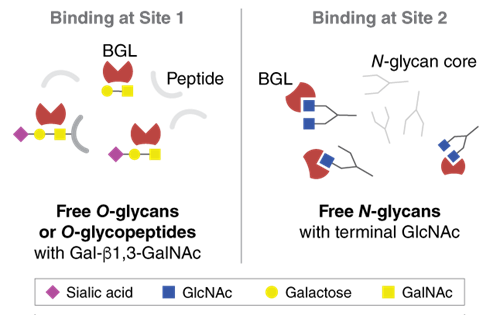
Boletopsis grisea Lectin (BGL) is a recombinant 15 kDa lectin from the Boletopsis grisea mushroom that has been expressed in E. coli. BGL has two separately functioning ligand binding sites (1). Site 1 binds to O-glycans bearing the Tn antigen (GalNAc-α-Ser/Thr) or Thomsen-Friedenreich antigen (TF-antigen; Gal-β1,3-GalNAc-α-) and Site 2 binds N-glycans with terminal GlcNAc residues.
- 产品类别:
- Glycobiology Products
- 应用:
- Glycobiology & Proteomics
-
产品组分信息
本产品提供以下试剂或组分:
NEB # 名称 组分货号 储存温度 数量 浓度 -
P0867S -20 Boletopsis grisea Lectin (BGL) P0867SVIAL -20 1 x 1 ml 1 mg/ml
-
-
特性和用法
贮存溶液
50 mM Tris-HCl
200 mM NaCl
pH 7.5 @ 25°C热失活
否
分子量
理论上的: 15 kDa
单位活性检测条件
100 µg BGL enriches >85% Procainamide-G0/A2 N-glycan in an Epitope Directed Glycan Enrichment (EDGE) assay (1,2). The assay involves an enrichment protocol termed EDGE whereby 100 µg BGL lectin is incubated with 100 ng Procainamide-G0 N-glycan substrate for 1.5 hours at 25°C in 20 mM Tris-HCl pH 7.5 buffer in a total volume of 120 µl.
-
相关产品
相关产品
- p8107-proteinase-k-molecular-biology-grade
- P8111 Thermolabile Proteinase K
- O-糖苷酶 & 神经氨酸苷酶套装
- β1-4 半乳糖苷酶 S
- α1-3,4,6 半乳糖苷酶
- Rapid 快速 PNGase F
- Trypsin-ultra™, Mass Spectrometry Grade
-
注意事项
- BGL has only been tested for the enrichment of O-glycopeptides and free N-glycans in EDGE assays.
- Optimal incubation time (60 to 90 minutes) will depend on the specific substrate.
- A lower starting amount of BGL for the EDGE assay could be used for O-glycopeptides. This amount should be empirically determined.
- The formulation of BGL contains 50 mM Tris-HCl, pH 7.5 and 200 mM NaCl. If this salt concentration will affect downstream workflow (e.g., capillary electrophoresis analysis), buffer exchange treatment of BGL may be performed prior to proceeding with the enrichment reaction.
-
参考文献
- Ganatra, M. B. et al. (2021). A bi‑specific lectin from the mushroom Boletopsis grisea and its application in glycoanalytical workflows. Sci. Rep. 11, 160.
- Vainauskas, S. et al. (2016). Profiling of core fucosylated N-glycans using a novel bacterial lectin that specifically recognizes α1,6 fucosylated chitobiose. Sci. Rep. 6, 34195.
操作说明、说明书 & 用法
-
操作说明
- Protocol for Enrichment of O-glycopeptides or N-glycans with terminal GlcNAcs with Boletopsis grisea Lectin (BGL) (NEB #P0867)
- A Typical Epitope Directed Glycan Enrichment (EDGE) Profiling Protocol using Boletopsis grisea Lectin (BGL) (NEB #P0867)
FAQs & 问题解决指南
-
FAQs
- Can I purchase large amounts of an existing Enzyme for Innovation?
- What are Enzymes for Innovation?
