上海金畔生物科技有限公司代理New England Biolabs(NEB)酶试剂全线产品,欢迎访问官网了解更多产品信息和订购。
产品信息
Spin columns containing an affinity matrix for the small-scale isolation and purification of polyhistidine-tagged (His-tagged) fusion proteins. Immobilized Metal Affinity Chromatography (IMAC) purifications employing NEBExpress® Ni Spin columns can be performed under native or denaturing conditions, yielding highly pure target in a single protein purification step. This enables screening of expression conditions and streamlines the functional and structural characterization of the target protein.
Support Matrix: Spherical, agarose based microparticles ranging in size from 10-100 μm.
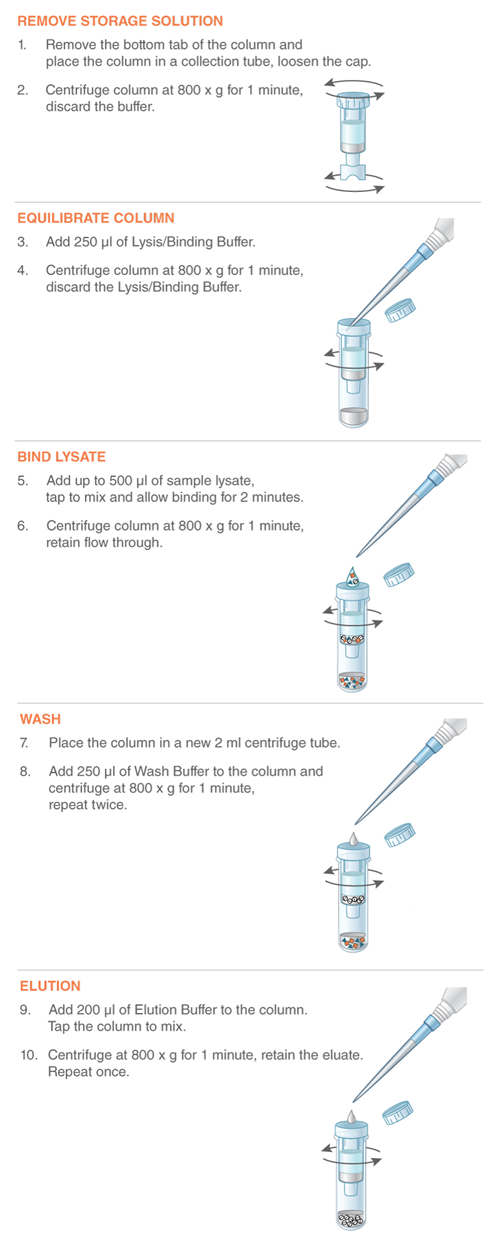
- 产品类别:
- Amylose Purification (MBP-tag),
- Nickel Purification (His-tag),
- Affinity Purification Products,
Protein Purification Products
- 应用:
- MBP Affinity Tag,
- Fusion Protein Cleavage,
- Pull Down Assays,
- His-tagged Protein Expression & Purification,
- Affinity Purification & Expression Tags,
- Protein Purification,
Protein Analysis Tools
-
产品组分信息
本产品提供以下试剂或组分:
NEB # 名称 组分货号 储存温度 数量 浓度 -
S1427S 4 NEBExpress® Ni Spin Columns S1427SVIAL 4 10 x 1 Each
(0.1 ml)Not Applicable 2X IMAC Buffer B1076SVIAL 4 1 x 30 ml 2 X 2M Imidazole B1077SVIAL 4 1 x 2 ml 2 M
-
S1427L 4 NEBExpress® Ni Spin Columns S1427SVIAL 4 25 x 1 Each
(0.1 ml)Not Applicable 2X IMAC Buffer B1076SVIAL 4 2 x 30 ml 2 X 2M Imidazole B1077LVIAL 4 1 x 10 ml 2 M
-
-
特性和用法
贮存溶液
20% Ethanol
支持介质
Spherical, agarose based microparticles ranging in size from 10-100 μm.
结合容量
Varies with target, ≥ 1 mg His-tagged fusion protein per column.
-
优势和特性
Features
- Isolation and purification of His-tagged fusion proteins under native or denaturing conditions as IMAC tolerates a wide range of conditions, including the presence of protein denaturants, reducing agents and detergents.
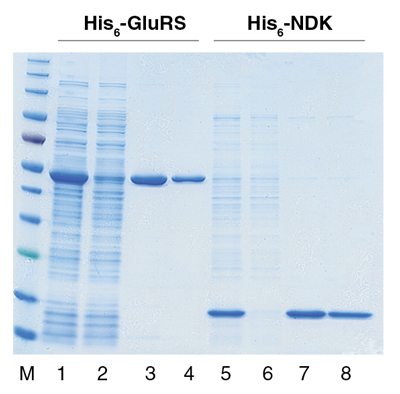
- High specific binding of His-tagged proteins from various expression systems yielding milligram quantities of target protein with purities of >95%.
- Can be used with common cell lysis reagents and a variety of buffer additives
-
相关产品
相关产品
- NEBExpress® Ni-NTA 磁珠
- TEV Protease
- Amylose Resin
- Amylose Resin High Flow
- 几丁质树脂
- NEBExpress® Ni Resin
-
注意事项
- Do not freeze.
- Several E. coli proteins are capable of binding immobilized metal affinity matrices with moderate to high affinity. The most common contaminants include SlyD (28kDa), GlmS (67kDa), ArnA (74kDa) and carbonic anhydrase (25 kDa) (1). The named contaminants may be eliminated from the Ni column elution fraction by employing NiCo21(DE3) (NEB# C2529H) as the expression host and then performing a second binding step with chitin magnetic beads (NEB# E8036) (2).
- Protein yield and purity are dependent upon the expression level, conformation and solubility characteristics of the recombinant fusion protein. For best results, estimate the expression level of the His-tagged protein of interest by first running a sample of the crude lysate on an SDS-PAGE gel.
- Unlike most IMAC resins, the unique chemistry employed in NEBExpress™ Ni Resin provides chemical tolerance to chelating agents such as EDTA and reducing agents such as DTT. Please consult the chemical compatibility table for more information.
- Polyhistidine tags do not typically compromise the biological protein function and are not considered immunogenic, however if cleavage of the His-tag is necessary we recommend using TEV Protease (NEB# P8112).
- Ni resin yields highly variable binding capacities dependent on the target and conditions. The binding capacity value (≥ 1 mg/column) for this product was determined by performing a mock purification from crude cell lysates expressing a His-tagged target protein.
-
参考文献
- Bolanos-Garcia et al (2006). BBA. 1760, 1304-1313.
- Samuelson, J (2016). GEN. 26-27.
操作说明、说明书 & 用法
-
操作说明
- NEBExpress® Ni Spin Columns Quick Start Protocol (NEB #S1427)
- NEBExpress® Ni Spin Column Reaction Protocol (NEB #S1427)
- His-tag removal from protein using TEV Protease
-
应用实例
- NEBExpress® Cell-free E. coli Protein Synthesis System
工具 & 资源
-
选择指南
- Purification Beads, Columns and Resins
FAQs & 问题解决指南
-
FAQs
- How much lysate can be loaded onto a single NEBExpress Ni Spin Column?
- Will extended binding increase the yield of the target protein in the eluate?
- What is the minimum recommended load volume?
- Is it necessary to cap the column during each centrifugation step?
- Why is the liquid not completely removed during the centrifugation step?
- I’ve prepared my lysate with too much buffer and the target is very dilute, can I apply more lysate by repeating the load steps?
- How can I reduce contaminating proteins in a Ni spin column protein purification?
- What are the recommendations for pH based elution, as opposed to imidazole?
- How can I remove imidazole from a protein sample?
- What are to the advantages of performing purification under denaturing conditions?
- How can I determine if my target protein remains bound to the resin?
- Why is imidazole not necessary in the lysis/binding buffer? Why does the wash buffer contain only 5mM imidazole?
-
问题解决指南
- Troubleshooting Guide for Purification using NEBExpress® Ni Spin Columns

