上海金畔生物科技有限公司代理New England Biolabs(NEB)酶试剂全线产品,欢迎访问官网了解更多产品信息和订购。
产品信息
E. coli 化学感受态细胞适用于多种应用中的高效转化。
重点
- DH10B™ 衍生菌株
- 可高效转化真核生物来源的甲基化 DNA,或来自 PCR、cDNA 和其它来源 [mcrAΔ(mrr-hsdRMS-mcrBC)] 的非甲基化 DNA
- 去除了非特异性核酸内切酶 I(endA1)的活性,以获得最高质量的质粒制备
- 抗 T1 噬菌体(fhuA)
- 适用于 b-半乳糖苷酶基因的 a-互补蓝白斑筛选(无 IPTG)
- 减少克隆 DNA 的重组(recA1)
- 可克隆大质粒和 BAC
- 无动物来源
- K12 菌株
基因型
Δ(ara-leu) 7697 araD139 fhuA ΔlacX74 galK16 galE15 e14- ϕ80dlacZΔM15 recA1 relA1 endA1 nupG rpsL (StrR) rph spoT1 Δ(mrr-hsdRMS-mcrBC)
- 产品类别:
- Cloning Competent Cell Strains Products
- 应用:
- USER® Cloning,
- Applications of USER® and Thermolabile USER II Enzymes,
- Site Directed Mutagenesis,
- Site Directed Mutagenesis,
- High-throughput cloning and automation solutions,
Transformation
-
产品组分信息
本产品提供以下试剂或组分:
NEB # 名称 组分货号 储存温度 数量 浓度 -
C3019H -80 NEB® 10-beta/Stable Outgrowth Medium B9035SVIAL 4 1 x 25 ml Not Applicable pUC19 Vector N3041AVIAL -20 1 x 0.025 ml 50 pg/µl NEB® 10-beta Competent E. coli (High Efficiency) C3019HVIAL -80 20 x 0.05 ml Not Applicable
-
C3019I -80 NEB® 10-beta/Stable Outgrowth Medium B9035SVIAL 4 1 x 25 ml Not Applicable pUC19 Vector N3041AVIAL -20 1 x 0.025 ml 50 pg/µl NEB® 10-beta Competent E. coli (High Efficiency) C3019IVIAL -80 6 x 0.2 ml Not Applicable
-
C3019P -80 pUC19 Vector N3041AVIAL -20 1 x 0.025 ml 50 pg/µl NEB® 10-beta/Stable Outgrowth Medium B9035SVIAL 4 1 x 25 ml Not Applicable NEB® 10-beta Competent E. coli (High Efficiency) C3019PVIAL -80 1 x 96-well plate
(20 μl/well)Not Applicable
-
-
特性和用法
Antibiotic for Plasmid Selection
用于质粒筛选的抗生素 使用浓度 Ampicillin 100 µg/ml Carbenicillin 100 µg/ml Chloramphenicol 33 µg/ml Kanamycin 30 µg/ml Tetracycline 15 µg/ml 货运单
- Ships on dry ice
抗生素耐药性
- str
-
优势和特性
Features
- 克隆大质粒和 BAC
- DH10B™ 衍生菌株
应用特性
质粒大小对转化效率的影响: 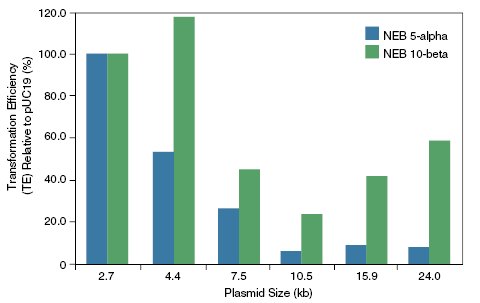
化学法制备的 NEB 10-beta 感受态细胞(C3019H)比 NEB 5-alpha 细胞(C2987H)更适合于大质粒的转化。质粒越大,两种感受态细胞的转化效率的差值就越大。 热激时间对 NEB 10-beta E. coli 感受态细胞转化效率的影响: 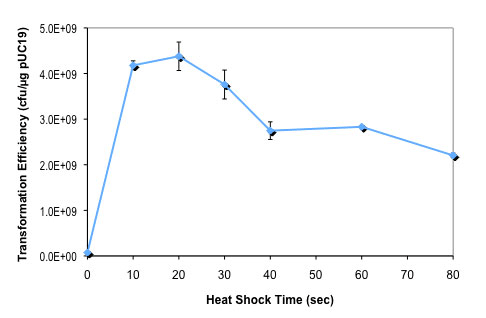
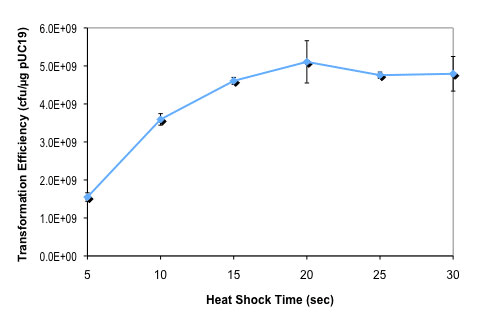 按照所提供的高效级转化操作流程,用 100 pg pUC19 对照 DNA 转化 50 μl 感受态细胞(C3019H),仅热激时间从 0 至 80 秒不等。
按照所提供的高效级转化操作流程,用 100 pg pUC19 对照 DNA 转化 50 μl 感受态细胞(C3019H),仅热激时间从 0 至 80 秒不等。DNA 温育时间对 NEB 10-beta E. coli 感受态细胞转化效率的影响: 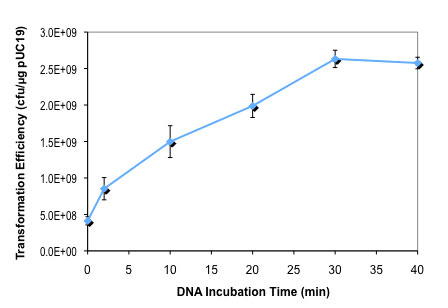
按照所提供的高效级转化操作流程,用 100 pg pUC19 对照 DNA 转化 50 μl 感受态细胞(C3019H),仅 DNA 温育时间为 0 至 40 分钟不等。 细胞复苏培养基对转化效率的影响: 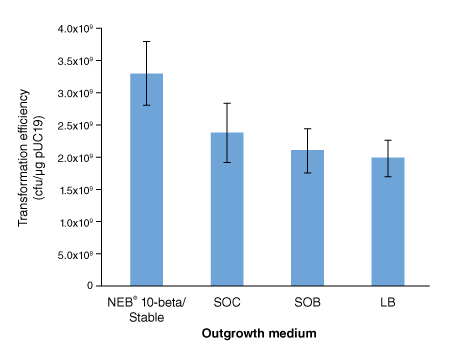
按照所提供的高效级转化操作流程,用 100 pg pUC19 对照 DNA 转化 50 μl 感受态细胞(C3019H),仅使用的复苏培养基不同。NEB 10-beta/Stable Outgrowth 培养基的转化效率最高。 NEB 10-beta(C3019H/I)高转化效率带来的巨大优势: 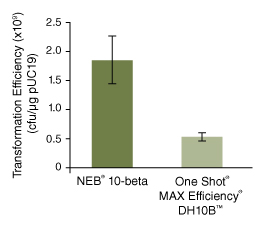
使用不同厂家的产品,按照所提供的产品说明书操作,比较转化效率。图中数值取自三次平行实验的平均值。 -
相关产品
单独销售的组分
- NEB® 10-beta/Stable Outgrowth 培养基
-
注意事项
- 贮存和处理:感受态细胞应在 -80℃ 条件下贮存。-20℃ 条件下贮存会显著降低转化效率。当细胞温度高于 -80℃ 时,即使不融化,细胞也会失去效率。
- 注意:本产品含危险物质 DMSO。处理前请先查看 MSDS。
操作说明、说明书 & 用法
-
操作说明
- High Efficiency Transformation Protocol using NEB 10-beta Competent E. coli (High Efficiency) (C3019H/C3019I)
- 5 Minute Transformation Protocol using NEB10-beta Competent E. coli (C3019H/C3019I)
- High Efficiency Transformation Protocol with NEB 10-beta in 96-well Plate Format (NEB# C3019P)
-
使用指南
- Additional E. coli Strain Genotypes
- Chemical Transformation Tips
- Genetic Markers
- McrA, McrBC and EcoKI Strain Phenotypes
- Restriction of Foreign DNA by E. coli K-12
-
应用实例
- Enhancing Transformation Efficiency
工具 & 资源
-
选择指南
- Characteristics of Select E.coli Strains
- Competent Cell Product Comparison
- Competent Cell Selection Guide
-
Web 工具
- Competitor Cross-Reference Tool
- NEBcloner®
FAQs & 问题解决指南
-
FAQs
- Which competent cell strains are compatible with Gateway® Cloning?
- What are the strain properties (C3019)?
- What is the difference between NEB #C3019H and NEB #C3019I?
- What is the shelf life for this strain (NEB #C3019H and NEB #C3019I)?
- Which strain of Competent E.coli should I use for general cloning?
- Does plasmid size affect transformation efficiency (C3019)?
- How should I calculate the transformation efficiency (C3019)?
- Can I store competent cells at -20°C instead of -80°C?
- What is the optimal heat shock time for this strain (NEB #C3019H and NEB #C3019I)?
- How long should I incubate cells on ice after DNA has been added (NEB #C3019H and NEB #C3019I)?
- Which kind of transformation tubes should be used?
- What volume of DNA can be added into competent cells?
- Are NEB’s competent cells compatible with the “Mix & Go” protocol?
- What type of competent cells are suitable for transformation of DNA constructs created using NEBuilder HiFi DNA Assembly Master Mix?
- Why did Synthetic Biologist Chris Voigt of MIT choose NEB 10-beta for DNA assembly and cloning?
- How should I store the NEB 10-beta/Stable Outgrowth Medium?
- How should fragments be prepared for assembly using NEBuilder HiFi?
- Can the 96-well plate format of NEB 10-beta Competent E.coli, NEB #C3019P, be separated into smaller sections?
- How does the transformation efficiency of the 96-well plate format (NEB #C3019P) compare to the other formats?
