上海金畔生物科技有限公司代理New England Biolabs(NEB)酶试剂全线产品,欢迎访问官网了解更多产品信息和订购。
产品信息

The Monarch RNA Cleanup Kit (10 µg) rapidly and reliably purifies up to 10 μg of concentrated, high-quality RNA (> 25 nt) from enzymatic reactions including labeling, capping, in vitro transcription (IVT) and DNase I treatment. This kit utilizes a bind/wash/elute workflow with minimal incubation and spin times. Our unique column design ensures zero buffer retention and no carryover of contaminants, enabling elution of sample in volumes as low as 6 μl. Eluted RNA is ready for use in a variety of downstream applications, including RT-PCR, RNA Library Prep for NGS and RNA labelling. The protocol can also be modified to enable the purification of smaller RNA fragments (≥ 15 nts).
Designed with sustainability in mind, Monarch kits use significantly less plastic and responsibly-sourced, recyclable packaging.
Monarch RNA Cleanup kits are also available for 50 µg (NEB #T2040) and 500 µg (NEB #T2050) binding capacities. Columns and buffers are also available separately for convenience.
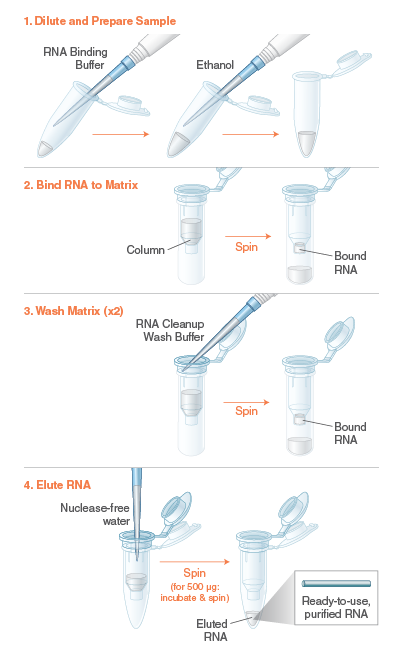
Specifications and Applications:
| SPECIFICATIONS | |
|---|---|
| RNA Sample Type | Cleanup and concentration of RNA from enzymatic reactions (labeling, capping, in vitro transcription reactions, DNase I treatment) |
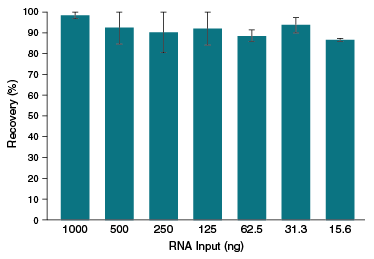
| Binding Capacity | 10 μg |
| RNA Size Range | ≥ 25 nt ( ≥ 15 nt with modified protocol) |
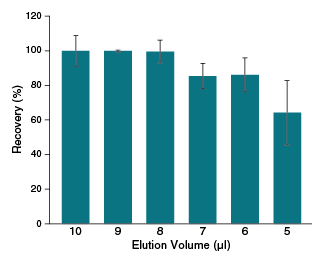
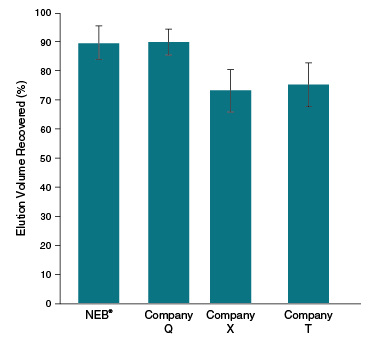
| Typical Recovery | 70–100% |
| Elution Volume | 6–20 µl |
| Purity | A260/280 > 1.8 and A260/230 > 1.8 |
| Protocol Time | 5 minutes of spin and incubation time |
| Compatible Downstream Applications | RT-PCR, Small RNA library prep for NGS, RNA Library Prep for NGS |
| APPLICATIONS | |
|---|---|
| RNA Cleanup and Concentration (including from the TRIzol aqueous phase) | RNA purified by other methods can be further purified |
| Enzymatic Reaction Cleanup | Enzymes such as RNA polymerases, DNase I, Proteinase K and phosphatases are removed allowing efficient desalting |
| In vitro Transcription Cleanup | Enzymes and excess NTPs are removed to yield highly pure synthesized RNA |
| RNA Gel Extraction | Purification of RNA from agarose gels |
| RNA Fractionation | Fractionation of RNA into small and large RNA pools |
- 产品类别:
- RNA Cleanup Products,
- RNA Extraction and Purification,
- Nucleic Acid Purification Products
-
试剂盒组成
本产品提供以下试剂或组分:
NEB # 名称 组分货号 储存温度 数量 浓度 -
T2030S 25 Monarch® RNA Cleanup Columns (10 μg) T2037-21 25 1 x 10 columns Not Applicable Monarch® Collection Tubes II T2018-21 25 1 x 10 tubes Not Applicable Monarch® Buffer BX T2041-21 25 1 x 3 ml Not Applicable Monarch® Buffer WX T2042-21 25 1 x 2.5 ml Not Applicable Nuclease-free Water B1500-21 25 1 x 2.5 ml Not Applicable
-
T2030L 25 Monarch® RNA Cleanup Columns (10 μg) T2037-1 25 2 x 50 columns Not Applicable Monarch® Collection Tubes II T2018-1 25 2 x 50 tubes Not Applicable Monarch® Buffer BX T2041-2 25 1 x 40 ml Not Applicable Monarch® Buffer WX T2042-2 25 2 x 20 ml Not Applicable Nuclease-free Water B1500-2 25 1 x 25 ml Not Applicable
-
操作说明、说明书 & 用法
-
操作说明
- Monarch® RNA Cleanup Kit Protocol
- Purification of RNA from the Aqueous Phase Following TRIzol®/Chloroform Extraction using the Monarch® RNA Cleanup Kits
- Separation of Large and Small RNA into Fractions using the Monarch® RNA Cleanup Kits
- Extraction of RNA from Agarose Gels using the Monarch® RNA Cleanup Kits
- RNA Extraction from Cells Using the Monarch RNA Cleanup Kits
- RNA Extraction from Buccal/Nasopharyngeal Swabs Using the Monarch RNA Cleanup Kits
-
说明书
产品说明书包含产品使用的详细信息、产品配方和质控分析。- manualT2030_T2040_T2050
-
使用指南
- Avoiding Ribonuclease Contamination
- Guidelines for RNA Quantitation
- Guidelines for Working with RNA During RNA Cleanup
-
应用实例
- A Practical Guide to Analyzing Nucleic Acid Concentration and Purity with Microvolume Spectrophotometers
- Purification of synthetic SARS-CoV-2 viral RNA from biological samples using the Monarch® Total RNA Miniprep Kit and the Monarch RNA Cleanup Kit
FAQs & 问题解决指南
-
FAQs
- What is the composition of each buffer provided with the Monarch RNA Cleanup Kits?
- What is the maximum binding capacity of the Monarch RNA Cleanup Column provided with the Monarch RNA Cleanup Kit?
- What is the smallest volume of nuclease-free water that can be used for elution with the Monarch RNA Cleanup Columns?
- Can I get better recovery with the Monarch RNA Cleanup Kits if I do a second elution with my eluent from the first elution?
- What factors affect my (A260/A230) when using the Monarch RNA Cleanup Kits?
- What size RNA can be purified with the Monarch RNA Cleanup Kit?
- Can I use the Monarch RNA Cleanup Kit to cleanup up my DNase I-treated RNA?
- Do you have a protocol for separating small and large RNAs into separate fractions?
- Can I use the Monarch RNA Cleanup Kits to purify RNA from agarose gels?
- Can I use the Monarch RNA Cleanup Kits to cleanup RNA after a TRIzol®/chloroform extraction?
- Are the Monarch RNA Cleanup Kits (NEB # T2030, #T2040, #T2050) compatible with Luna RT-qPCR reagents?
- Are the Monarch RNA Cleanup Kits (NEB #T2030, #T2040, #T2050) compatible with NEBNext reagents for RNA library prep?
- How can I assess RNA integrity and purity?
- Are the columns in the Monarch RNA Cleanup Kits the same as those in the Monarch Total RNA Miniprep Kit (NEB #T2010)?
- Can I purchase Monarch buffers and columns separately?
- Can I do an on-column DNase I treatment with the Monarch RNA Cleanup Columns?
- Can the Monarch RNA Cleanup Kits (NEB #T2030, #T2040, #T2050) also be used to purify DNA?
- Can the Monarch RNA Cleanup Kits be used for RNA extraction?
- I noticed the buffer names have changed in the RNA Cleanup Kits. Are these buffers the same formulation as before?
-
问题解决指南
Problem Cause Solution Low RNA Yield Reagents added incorrectly Check protocol to ensure correct buffer reconstitution, order of addition of buffers and ethanol, and proper handling of column flow-through and eluents. Insufficient mixing of reagents Ensure the ethanol is thoroughly mixed with RNA sample and RNA Cleanup Binding Buffer before applying the sample to the RNA Cleanup Column. Incomplete elution during prep Ensure the nuclease-free water used for elution is delivered directly to the center of the column so that the matrix is completely saturated. Larger elution volumes, multiple elutions, and longer incubation times can increase yield of RNA, but will dilute the sample and may increase processing times. For typical RNA samples, the recommended elution volumes and incubation times should be sufficient. High degree of RNA secondary structure Binding and elution of smaller RNAs (< 45 nt) can be affected by secondary structure of the RNA molecules. If poor yield of a small RNA is observed, we recommend diluting your sample with 2 volumes of ethanol instead of one volume in Step 2 of the protocol. Purified RNA is Degraded RNase contamination In order to avoid RNase contamination during RNA cleanup, make sure to work on a clean lab bench, wear gloves and use disposable RNase-free pipet tips and microfuge tubes (not provided). Keep all kit components tightly sealed when not in use. Improper storage of RNA Purified RNA should be used immediately in downstream applications or stored at -70°C. Low A260/230 Ratios Residual guanidine salt carry-over Ensure wash steps are carried out prior to eluting sample. Use care to ensure the tip of the column does not contact the flow-through. If unsure, repeat centrifugation. When reusing collection tubes, blot the rim of the tube on a Kimwipe prior to reattachment to the column to remove any residual wash buffer. Low Performance of RNA in Downstream Steps Salt and/or ethanol carry-over Ethanol and salt remaining after the washes may inhibit downstream applications. Use care to ensure that the tip of the column does not come into contact with the flow-through. If in doubt, re-centrifuge for 1 minute to ensure traces of salt and ethanol are not carried over in the eluted RNA. DNA contamination DNA removal may be necessary for certain applications. Incubate RNA sample with DNase I (NEB #M0303) and cleanup RNA using the Monarch RNA Cleanup Protocol. - Troubleshooting Guide for RNA Cleanup
